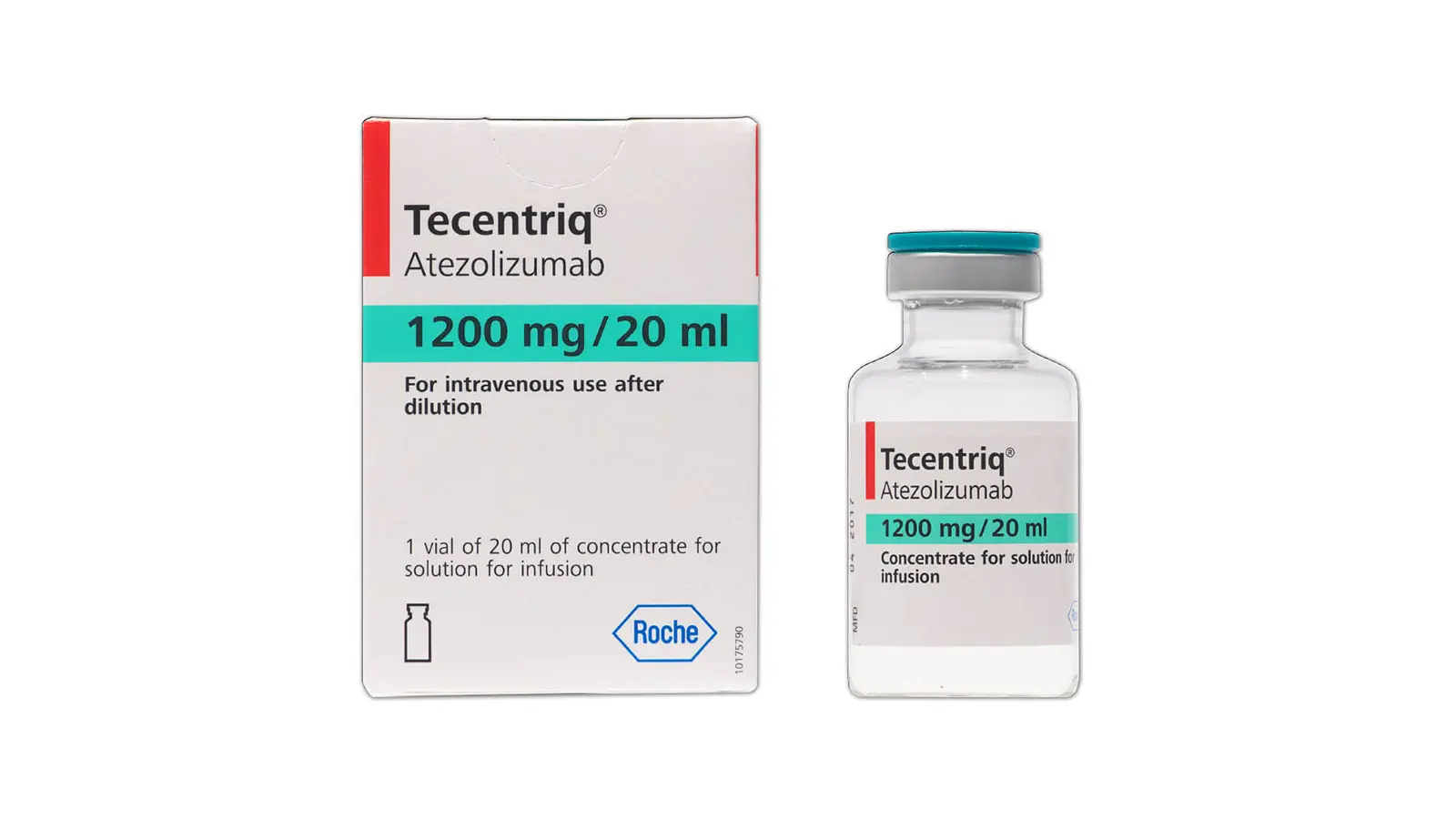
TECENTRIQ-1200MG INJ
₹396,725.00 Original price was: ₹396,725.00.₹346,650.00Current price is: ₹346,650.00.
Composition : ATEZOLIZUMAB
Manufacturer : PFIZER
Origin of Medicine : SWITZERLAND
Packing : 1’s Vial
Requires Prescription
Category: Anti-Cancer Tags: Anti-Cancer, AntiCancer, ATEZOLIZUMAB, Injection, Oncology, Price, Rate, TecentriqDescription
TECENTRIQ-1200MG INJ
Atezolizumab is is a programmed cell death ligand 1 (PD-L1) blocking antibody. Atezolizumab is an Fc-engineered, humanized, non-glycosylated IgG1 kappa immunoglobulin that has a calculated molecular mass of 145 kDa.
TECENTRIQ Injection for intravenous use is a sterile, preservative-free, colorless to slightly yellow solution in single-dose vials. Each mL of TECENTRIQ contains 60 mg of atezolizumab and is formulated in glacial acetic acid (16.5 mg), L-histidine (62 mg), sucrose (821.6 mg), polysorbate 20 (8 mg), pH 5.8.
Uses of Tecentriq 1200mg Injection
- Urothelial carcinoma
- Non-small cell lung cancer
- Triple-negative breast cancer
- Hepatocellular carcinoma
- small cell lung cancer
Side Effects of Tecentriq 1200mg Injection
Serious
- Inflammation (lung, liver, intestine, thyroid, adrenal glands, and pituitary gland)
- Type 1 diabetes
- Severe skin reaction
- Inflammation of problem with nerve
- Inflammation of the brain, heart muscle, kidney, muscle
Common
- Fever
- Loss of appetite
- Numbness in hands and feet
- Sensitivity to sun
- Constipation
- Urinary tract infection
- Pain in the joints, muscles, and bones
- Headache
- Low oxygen level
- High blood sugar
- Hair loss
- Pain in stomach, mouth, and throat
Related
Be the first to review “TECENTRIQ-1200MG INJ” Cancel reply
You must be logged in to post a review.
What is the valid prescription?
Related products
Anti-Cancer
Anti-Cancer
Anti-Cancer
Anti-Cancer
Anti-Cancer
Anti-Cancer
Anti-Cancer
Anti-Cancer


Reviews
There are no reviews yet.